Untitled Document
Photobiology of the retina covers broad aspects of the phototransduction cascade responsible for visual perception, as well as the pupillary light reflex, and the role of the retina in setting up our circadian rhythms. All of these functions of the retina depend on the absorption of photons. However, excessive exposure to light results in damage to the retina. The phototransduction cascade is discussed by Oyster in "Retina I: Photoreceptors and Functional Organization". Here we will review current understanding of light-induced damage to the retina. As the visual cycle plays an important role in susceptibility of the retina to light damage, certain aspects of it will be discussed here in more detail.
Types of Light-Induced Damage to the Retina
Throughout life, the eye is exposed to daily fluxes of solar radiation. Solar radiation is filtered by the Earth's atmosphere so that at sea level about 80% of the solar energy is restricted to a narrow spectral band from about 300 nm in the ultraviolet to 1100 nm in the infrared. Longer wavelengths are primarily filtered out by atmospheric water vapour, whereas shorter wavelengths are absorbed by the ozone layer. Furthermore, certain spectral components of solar light incident on the cornea are partially filtered out before reaching the human retina (1) (Figure 1). The cornea absorbs wavelengths below 295 nm while the lens in the adult human eye absorbs strongly longer-wavelength UVB (295-315 nm), and the full range of UVA (315-390 nm). Both the cornea and the lens also absorb part of the infrared radiation - mainly the water bands at 980 nm, 1200 nm, and 1430 nm. The vitreous absorbs light above 1400 nm, up to 10  m. Thus, the non-ionizing radiation reaching the retina is the so-called 'visible component' of the electromagnetic spectrum (390-760 nm), and some of the near infrared (760-1400 nm).
m. Thus, the non-ionizing radiation reaching the retina is the so-called 'visible component' of the electromagnetic spectrum (390-760 nm), and some of the near infrared (760-1400 nm).
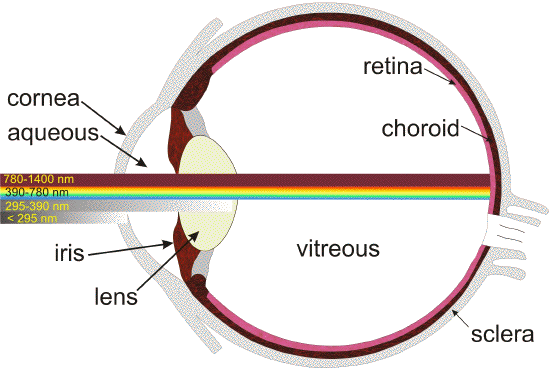
Figure 1. Transmission of light through the young adult human eye to the retina [modified from (289)].
In young children, some UV-B and UV-A can reach the retina, namely the spectral range of 300-340 nm, with a maximum of that
transmission window of about 8% at 320 nm (1). This transmission band is gradually reduced when metabolites of tryptophan absorbing UV light accumulate in the lens. By the age of 22 years, only 0.1%, and by the age of 60 years virtually no UV light reaches the retina except for aphakic individuals.
The transmission of visible light decreases with increasing age, and arises largely from age-related changes in the composition of the lens, which accumulates chromophores absorbing short-wavelength visible light. Lenses older than 70 years exhibit a relatively slow increase in transmittance with increasing wavelength: the transmission starts at about 400 nm, but does not reach the maximum until about 600 nm. Overall the transmission of visible light is significantly reduced in older lenses, especially in the blue region of the spectrum. Typical daily activities are related with exposures of the retina to light levels well below the threshold doses causing acute photodamage to the retina (Figure 2). However, direct gazing at the sun or artificial sources of intense visible or infrared light can easily lead to exceeding that threshold, and damage the retina.
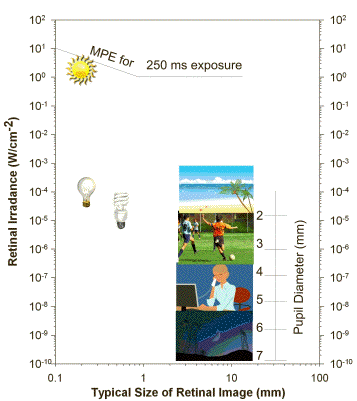
Figure 2. Typical retinal irradiance levels during common daily activities, retinal irradiance levels from different sources of light: the sun, frosted incandescent lamp, fluorescent lamp and typical size of their images on the retina for a 0.5 s exposure. The diagram also shows the maximal permissible exposure (MPE) for 0.25 s exposure of the eye with a 2 mm pupil and the dependence of pupil size on retinal irradiance. Modified from (5, 273).
Visible and infrared light reaching the retina can induce tissue damage via at least one of three fundamental processes: photomechanical (or photoacoustic), photothermal (photocoagulation) and photochemical, depending on its fluence rate, total dose and spectral characteristics.
Photochemical injury. Photochemical damage occurs when light is absorbed by a chromophore and leads to the formation of an electronically excited state of that molecule, which then undergoes either chemical transformation itself and/or interacts with other molecules leading to chemical changes of both interacting molecules or to a transfer of the excitation energy to the other molecules (Figure 3). Importantly, when photochemical damage occurs there is no substantial increase in temperature of the tissue. In a particular type of photochemical damage, photosensitized damage, the photoexcited chromophore in its electronically excited singlet state undergoes intersystem crossing and forms an excited triplet state (Figure 3). The excited triplet state is relatively long-lived, allowing for interaction with other molecules producing free radicals - via electron (hydrogen) transfer (type I of photosensitized damage), or singlet oxygen,

- via transfer of excitation energy from the photosensitizer in the triplet state to molecular oxygen (type II of photosensitized damage). Photosensitizers can act as catalysts of extensive damage where numerous free radicals and singlet oxygen molecules are generated by a single molecule of a photosensitizer, which is constantly recycled to its ground state (Figure 3B).
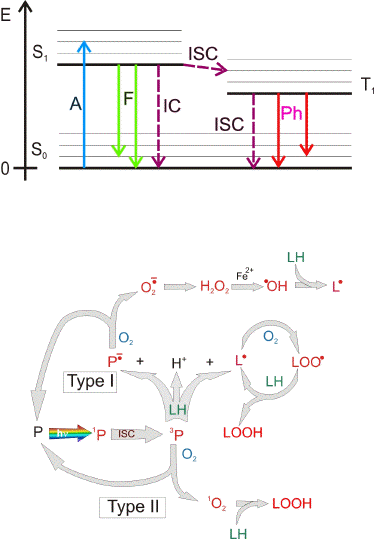
Figure 3. Jablonski diagram of photoexcitation of a molecule and 3 main deactivation pathways (Upper Figure). As most biologically relevant molecules are in a singlet state in their ground state (S0), their photoactivation leads to an electronically excited singlet state (S1): an electron from the highest occupied molecular orbital (HOMO) is transferred to the lowest unoccupied molecular orbital (LUMO). From that electronically excited singlet state there are 3 main pathways of deactivation: 1) thermal deactivation is a radiation-less process, called also internal conversion (IC) where the photoexcited molecule returns to the ground state releasing the excitation energy in a form of heat and no change in the molecular spin occurs; 2) fluorescence (F) where the photoexcited molecule returns to the ground state releasing the excitation energy in a form of an emitted photon; 3) intersystem crossing (ISC) where the photoexcited electron changes orientation of its spin resulting in a change in the multiplicity and formation of an excited triplet state (T1). The lifetime of an excited triplet state is usually in the range of microseconds and longer, that is at least 3 orders of magnitude longer than of an excited singlet state (in the range of ps-ns). An excited triplet state deactivates via radiation-less transition to the ground state via an intersystem crossing (ISC) or a release of photon known as phosphorescence (Ph).
Long lifetime of an excited triplet state increases the probability of interaction with other molecules (Lower Figure). Photoexcitation of a molecule (P) to an excited singlet state (1P) may be followed by an intersystem crossing and formation of an excited triplet state (3P). An excited triplet state (3P) can transfer an electron (or hydrogen) to/from another molecule leading to a formation of a radical pair (Type I of photosensitized damage). Interaction of an excited triplet state with molecular oxygen (which is in a triplet state in its ground state) may lead to an energy transfer (type II of photosensitized damage). As a result, the photoexcited molecule returns to its ground state while oxygen is activated to an excited singlet state, called singlet oxygen (1O2). Chromophores which upon photoexcitation undergo intersystem crossing and produce free radicals and singlet oxygen are known as photosensitizers (P). As a result of an interaction of the triplet state with an electron donor (LH), such as an unsaturated lipid, the formed radical anion of the photosensitizer may donate the electron to oxygen leading to the formation of superoxide radical anion (O2.-). The free radical formed from the electron donor after hydrogen abstraction (L.) can give rise to a free radical chain of peroxidation of biomolecules such as lipids and proteins. L. may interact with oxygen forming a peroxyl radical (LOO.). The peroxyl radical may abstract an electron/hydrogen from other molecules resulting in a formation of another L. and a hydroperoxide (LOOH). Hydroperoxides may become decomposed by redox active metal ions, such as iron, leading to the formation of more free radicals. A single molecule of a photosensitizer may produce numerous free radicals and singlet oxygen molecules as long as it is recycled to the ground state and photoexcited by subsequent photons.
Photosensitized damage mediated by oxygen (photodynamic damage) has been employed in photodynamic therapy (PDT) to destroy tumours and unwanted retinal neovascularisation. In PDT a photosensitizing drug is delivered to the tissue of interest followed by irradiation with an appropriate laser light to trigger the photodynamic damage. However, the retina contains a number of endogenous photosensitizers which can be excited by visible/infrared light reaching the retina. The outer retina [photoreceptors and retinal pigment epithelium (RPE)], is immediately adjacent to the choroidal blood supply and thus highly oxygenated. Therefore, these are potentially favourable conditions for photodynamic damage to occur. The strong dependence of susceptibility of the retina to photodamage on oxygen concentration suggests that light-induced damage to the retina is indeed photodynamic in nature (2-4). Photochemical damage usually demonstrates delayed onset following light exposure, and in the retina, this delay may take several hours.
Photothermal injury. Photothermal damage occurs when the rate of light energy deposition by thermal deactivation is faster than thermal diffusion, so the temperature of the exposed tissue rises (5). This is the case for exposure to intense flashes of light shorter than ~20

s when the heat diffusion can be neglected during the duration of the exposure so that the energy needed to produce retinal damage is independent of the exposure duration within that timeframe. For visible and infrared light reaching the retina, melanin and haemoglobin are the primary absorbers with an ability to undergo very efficient non-radiative decay from their electronically excited states to the ground state. Typically, when the rise in temperature is at least 10°C above the physiological temperature, then thermal damage occurs, which leads to thermal denaturation of many proteins.
Surgeons employ photophysical properties of melanin and haemoglobin as endogenous chromophores to cause thermal photocoagulation of retinal tissues to treat proliferative diabetic retinopathy, neovascular form of AMD or macular edema (6-9). Using visible or near infrared laser sources, they induce a therapeutic photocoagulation of unwanted blood vessels in the retina or by retinal photocoagulation prevent retinal detachment (10). The depth of penetration is dependent on the incident wavelength, e.g., optical radiation from argon lasers (457-524 nm) is primarily absorbed by haemoglobin and oxyhaemoglobin in retinal blood vessels, and melanin in the retinal pigment epithelium (RPE) while that from krypton red (around 650 nm) and diode lasers (790-830 nm) is absorbed by the RPE, as well as choroidal melanocytes and blood.
Photomechanical injury. Photomechanical (or photoacoustic) damage occurs when the light energy is deposited faster than mechanical relaxation can occur and typically occurs for intense pulses shorter than 1 ns (5). As a result, a thermoelastic pressure wave is produced, and tissue is disrupted by shear forces or by cavitation. Fluence rates needed to produce photomechanical damage can be obtained from sources such as intense pulse lasers.
Continue reading article ...
